The international needs for patients’ safety have led many countries to adopt some regulations for identifying the “Medical Devices” (contact lenses, bandages, scalpels, syringes, prosthesis, vitro diagnostic devices).
The UDI code (Unique Device Identifier) provides all the standardized information related to the life cycle of the device: from its production to its final use to prove its authenticity.
USA (with the Food and Drug Administration FDA regulation) and UE (with the Medical Devices Regulation MDR 2017/745 and IVDR 2017/746 regulations) have approved regulations for the identification of medical devices while in Canada, Japan, China, Brazil, South Korea, China, Israel, Saudi Arabia and South Africa are about to be defined.
HOW TO SERIALIZE MEDICAL DEVICES
The producers must apply specific Unique Device Identifiers codes on all distributed medical devices. UDI is a unique numeric or alphanumeric code consisting of two parts:
– Device ID (UDI-DI, Device Identifier). Identifies the specific version or model of the product (mandatory and fixed).
– Production identification (UDI-PI, Production Identifier). It is variable and can contain one or more of the following information:
- Batch number
- Date of production
- Expiry date
- Serial number
This information must be provided both in a human readable format (alphanumeric text) and in a format that can be read by machines using AIDC technology (Automatic Identification and Data Capture).
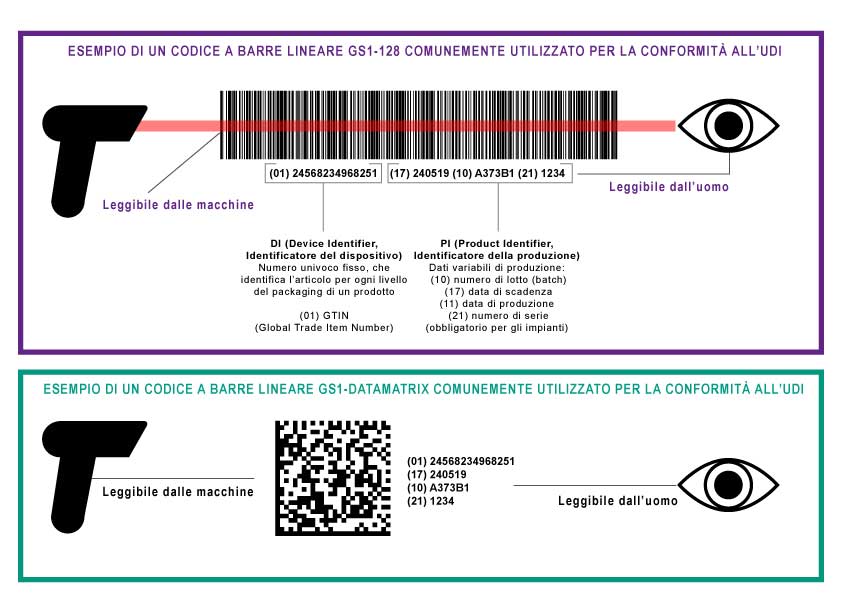
An UDI code must be placed on the primary packaging and at all upper levels of the device’s packaging. Since the GTIN (Global Trade Item Number, an identification number issued by GS1) is unique for each level of the device’s packaging, the UDI code is also unique to any level of the packaging.
In fact, direct marking allows constant identification over time even when a device is no longer associated with the label or packaging.
ARCA TECHNOLOGY FOR THE MARKING OF MEDICAL DEVICES

LASER MARKERS (UV, GREEN, FIBER, MOPA, CO2)
The ideal technology for direct marking on medical devices, indeed laser markers guarantees to:
– not damage the product preserving its integrity;
– etch high quality contents without defects, indelible and always readable;
– resist to highly alkaline, acid and corrosive cleaning processes.

PRINT APPLY
The Ideal technology for printing UDI code in real time on a label and applying it on a product or a secondary packaging or a pallet allowing the code aggregation process (creation of the parent-child relationship between the unique codes of the unit packets and the UDI codes of the subsequent packaging levels) with the aim to obtain a better traceability of the medical devices.
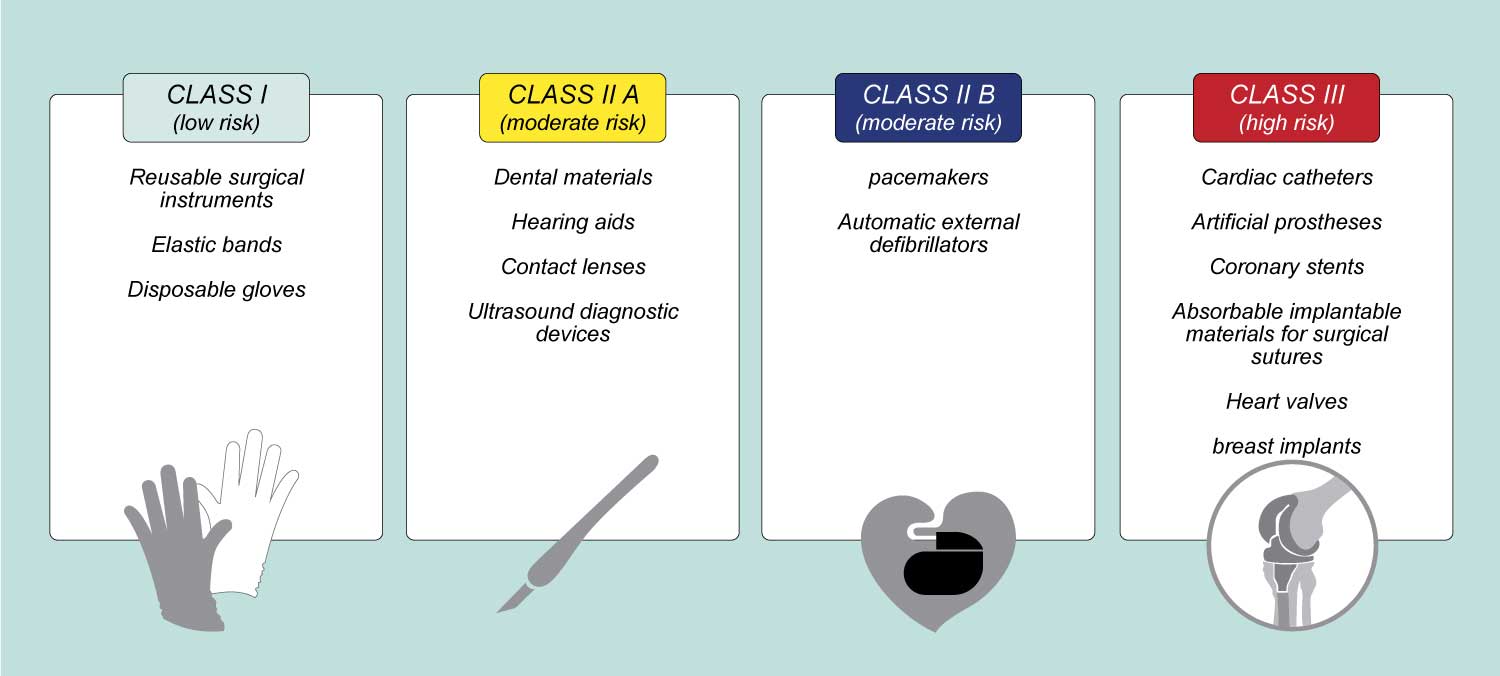
CLASSIFICATION OF MEDICAL DEVICES
The classification of the medical device is based on the level of risk associated with the device under examination.
- Class III devices: cardiac catheters, coronary stents, heart valves, breast implants, HIV diagnostic tests, endosseous implants, artificial prostheses, long-term contact lenses, absorbable implantable materials for surgical sutures …
- Class II devices b: Pacemakers, automatic external defibrillators, acupuncture needles, infusion pumps …
- Class II devices a: Daily contact lenses, motorized wheelchairs, surgical drapes, dental materials, hearing aids, ultrasound diagnostic devices …
- Class I devices: Elastic bandages, latex gloves, portable and reusable surgical instruments.